China conditionally approves four self-developed COVID-19 vaccines: official
Share - WeChat

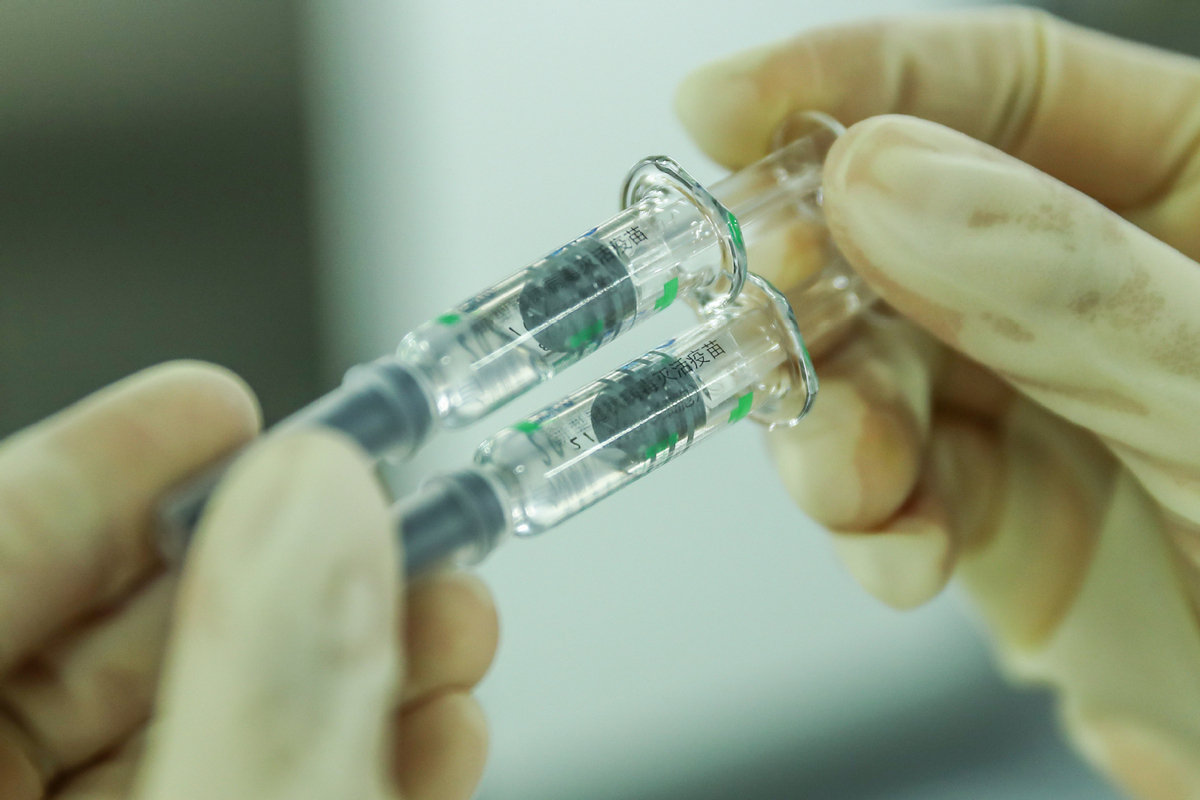
BEIJING - China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.
- Xizang's remote villages gain access to express delivery services
- Egyptian man explores 460-year-old fair in Tianjin
- 10,000-ton electric container ship tests off Jiangxi province
- China's construction sector sees drop in energy use, emissions during build phase, rise in operations
- Guangdong sees surge in holiday arrivals as travel pattern shifts
- International commercial court established in Guangzhou