Seven Chinese-made COVID-19 vaccines enter phase-3 clinical trials: official
Share - WeChat

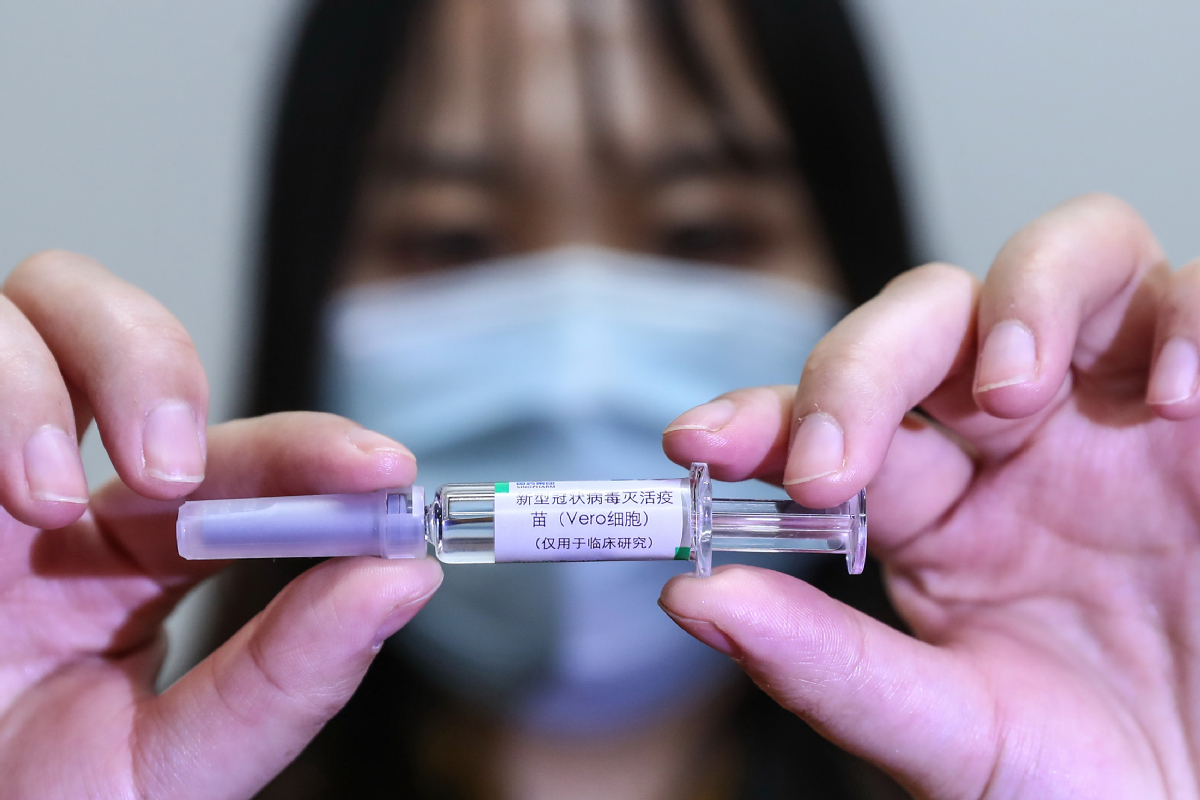
BEIJING - A total of seven Chinese-made COVID-19 vaccines have entered phase-3 clinical trials, according to an official with the Ministry of Science and Technology (MOST).
China now has 16 self-developed COVID-19 vaccines undergoing clinical trials, said Wu Yuanbin, director-general of science and technology for social development with MOST, at a recent conference on hematology.
An inactivated vaccine developed by Beijing Biological Products Institute Co Ltd under the China National Biotec Group (CNBG), affiliated with Sinopharm, was last month the first to receive conditional market approval from the National Medical Products Administration.
The interim results of its phase-3 clinical trials show 79.34 percent efficacy against COVID-19.
Related Stories
- China carries out key test on a new type of reusable carrier rocket
- PLA unit conducts nighttime drill simulating terrorist elimination
- China's light sports aircraft gains core independence with homegrown engine and avionics
- Record number of black-necked cranes arrive at Guizhou's nature reserve
- New morning and evening peak-hour trains to run between Beijing and Xiong'an
- College student rediscovers figure skating passion